
Written by Crista Casey, VP of Clinical and Regulatory Operations
First and foremost, patient safety is paramount.
Transitioning from pre-clinical to clinical trial work is an exciting time; ripe with adventure and possibilities. During this time, program level planning increases in detail and structure while individual projects take shape. Deliberate flexibility in program and project planning allows trials to excel in areas like regulatory submission and approvals, enrollment and retention, and data review. Simple language adjustments to the protocol, intelligent use of decentralized services and real time data visibility via intuitive technology like encapsia can reduce project timelines by weeks or even months.
Clinical trials have been described as a relay race with each trial period (such as start up or enrollment) being legs of the race with a cumulative effect from the catalyst event. Study start-up is the first leg of the relay race. Having experienced people and efficient processes to push forwards during this very busy time is important. However, the impact of that effort can be amplified by the effective use of technology in areas from electronic information collection, start-up and feasibility visualizations, to digital document signature and submission. This approach decreases effort and costs, while being easily applied in a decentralized environment.
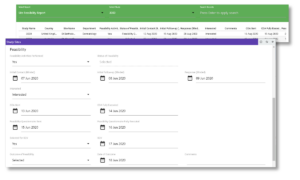
Figure 1: Centralized, web-based visibility into Feasibility via Cmed CTMS.
Screening and enrollment in early phase interventional trials is an exercise driven by planning and control. Diligence during feasibility informs enrollment projections and ensures we are starting with an achievable goal. Real time visibility into recruitment and conduct statuses from a central data source is integral to the control of this process.
Flexibility in the methods of enrollment (e.g. eConsent) and study assessments (e.g. onsite, near home, at home, decentralized data sources like eCOA or eDiary, telehealth, etc) offer a patient centric approach. Prior to Covid-19, these methods were shown to improve enrollment, retention and data quality. During the Covid-19 pandemic they became increasingly important and continue to be effective.
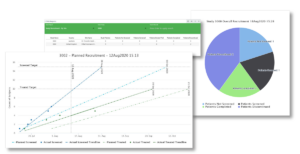
Figure 2: Sample enrollment related outputs from Cmed’s technology suite-encapsia and CTMS.
Decentralized data sources and remote access to source data are changing the model from Medical Monitoring, Clinical Monitoring and Data Management to what we call Integrated Data Review. We’re able to align specialists with specific types of data review and insights/visualizations for controlled, efficient and faster outcomes. Simply put, the data can be reviewed faster for stakeholder consumption. Plus, the use of encapsia real-time data access and insights minimizes the time to dose review committee decisions.
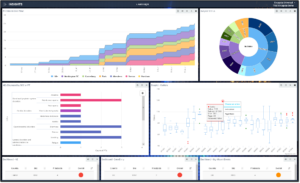
Figure 3: Samples of customizable, real-time insights from encapsia
Trial data is a very valuable element of clinical research. Without the data, there isn’t a treatment or cure. Ongoing data visibility and technology supported data cleaning processes permit earlier access to data that is complete, consistent and relevant in terms of ensuring patient safety and high-quality decision making.
Relevant experience:
- 400 Phase I/II studies
- 100 FIH Trials
- Average 20 years’ experience across the CRO
- Based in USA and Europe, with trials run globally
- Phase I/II adaptive designs
Encapsia visualizes the clinical data and trial metadata in real time, enabling trial reviewers to quickly identify missing or anomalous data instead of wasting time moving data between systems in batches for review and interrogation.
Cmed is a full service, global CRO. We have been approaching trials from a point of efficiency and technological solutions for over twenty years. Our cross-functional group of early phase experts enjoy strategy development. Let us help you build success into your product or project planning.
If you would like to talk to our team about our experience and capabilities in virtualized clinical trials, please contact us:
