
How We Apply 20 Years’ Oncology Trial Experience to Help Find New Therapies
An expert view by Crista Casey, VP, Clinical and Regulatory Operations
Glioblastoma, also known as glioblastoma multiforme, can be very difficult to treat and a cure is often not possible. It is an aggressive type of cancer that can occur in the brain or spinal cord and it can develop at any age, but tends to occur more often in older adults. The global incidence rate of Glioblastoma is <5 per every 100,000, but rising in some locations, and has an average survival rate of 14-15 months post diagnosis. Traditionally, survival from a GBM is less than a year, and patients typically live less than six months after recurrence. The graphs below present survival and incidence.
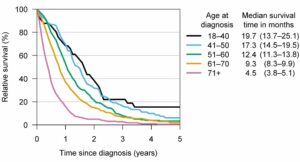
Median survival times and relative survival rates for different glioblastoma patient age groups. Credit: University of Helsinki
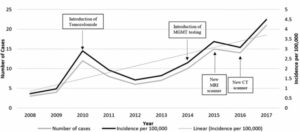
Cases and incidence of glioblastoma multiforme per year, with possible causal factors
Approximately five brain cancer approved treatment options are available in addition to radiation:
- Afinitor/Everolimus: Approved for subependymal giant cell astrocytoma patients with tuberous sclerosis
- Bevacizumab/Avastin: Conditionally approved for recurrent GBM
- Carmustine/Lomustine: Approved for glioma patients, and most often used in low-grade gliomas and in recurrent GBM patients
- Temozolomide/Temodar: Approved for newly diagnosed GBM
- Optune (NovoTTF-100A Device): Approved for recurrent and newly diagnosed GBM
Current data shows there are 121 products in development across 119 sponsors (commercial and academic). With a poor prognosis and limited number of therapies approved specifically for glioblastoma treatment, the indication is ripe for new therapy options. A little over 2,400 trials have been conducted in glioblastoma and 931 of those have been with marketed products. Globally, there are over 18,000 oncology trials currently enrolling or planned worldwide with just 456 enrolling or planned trials in glioblastoma.
Over the past decade, our neurologists, oncologists and clinical team experienced firsthand shifts in this research space from the use of GLIADEL Wafers, first approved devices and changes in preferred terminology e.g. glioblastoma vs glioblastoma multiforme to recent potential efficacy differences between Temodar and generics.
We understand the specific challenges in treating and researching this population and apply our medical and operational expertise when designing and implementing glioblastoma trials. For example, we consider the following issues with a sampling of mitigations from the clinical perspective:
- The choice imaging modality is MRI which isn’t always available and is expensive. Modalities should be considered during the design phase and should include input from regional care teams.
- Maximal safe surgical resection is a key for better survival.
- Resection may not be possible due to the location of the tumor. This can impact available populations or tissue biopsy related endpoints.
- Biopsy/minimal resection in lieu of resection (location driven). The extent of surgery (biopsy vs resection) has been shown in a number of studies to affect length of survival.
- Interstitial brachytherapy is of limited use and is rarely used.
- Post-surgical hemorrhage is a very real risk. We recommend post resection MRI’s to quickly identify post-surgical hemorrhage.
- High recurrence rates: Median time to recurrence after standard therapy is 6.9 months.
- Standard of Care is the same for methylate and unmethylated tumors, but unmethylated don’t respond to temozolomide the same way. The definitions of response in protocols need to accommodate this.
- Different performance expectations must be considered for newly diagnosed vs progressed patients. These populations behave differently and differences for response, quality of life, etc must be accommodated in the trial design.
- For patients older than 70 years less aggressive therapy is sometimes considered, using radiation or temozolomide alone. This can lead to differences in treatment across the study’s population and stratifications may be needed during statistical analysis.
- Radiotherapy and/or radiosurgery for recurrent GBM is controversial.
- Pseudoprogression should be confirmed in 4-6 weeks in order to re-evaluate and adjust the patient’s treatment.
Operationally, there are some administrative and support challenges such as:
- When temozolomide is given SOC with experimental treatment, dosing compliance for TMZ and drug accountability can be difficult to track. We mitigate this by understanding the site standard of care early in the study development to design complimentary tools or processes to collect this information.
- The patient’s decline e.g. recall, fall status and other cognitive changes are difficult for families and care teams. We see care giver mentoring and even home care support being successful in this area.
Cmed is one of only 44 Clinical Research Organizations (CRO) that have supported glioblastoma studies. The Cmed team has been working in glioblastoma research for a decade. We have successfully delivered 13 commercial trials in glioblastoma, including two successful FDA submissions. Our 14th glioblastoma study with an ATIMP is currently in startup and our 15th trial, also an ATIMP, is expected to start early in 2021.
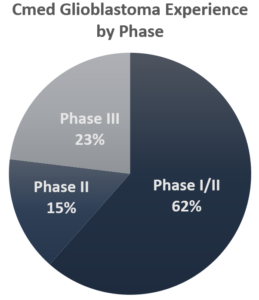
- Phases I-III
- 13 Trials in commercial research
- Newly diagnosed and progressed/recurrent populations
- Product types include inhibitors, peptides, vaccine
- Mono, combo and resection inclusive trial designs
- Global trials, including but not limited to US, Austria, Canada, Czech Republic, France, Germany, Israel, Italy, Japan, South Korea, Spain, Sweden and Switzerland.
- Trial size ranges are 1-89 sites and 30-700 patients
Cmed is a full service, global CRO. We have been approaching trials from a point of efficiency and technological solutions for twenty years. Let us help you build success into your product or project planning.
